- October 19, 2023
Panchali Roychoudhury
Introduction
In the world of clinical trials, achieving real-time end-to-end visibility has become more than just a trend; it’s a critical necessity. Modern clinical trials are complex endeavors involving numerous stakeholders, generating massive amounts of data that reside in disparate systems. To navigate this complexity and make informed decisions, pharmaceutical companies are turning to advanced data analytics and customized visual dashboards.
The Demand for End-to-End Visibility
Clinical trials are no longer isolated studies but rather complex ecosystems involving pharmaceutical companies, research organizations, regulatory bodies, and healthcare professionals. Each trial generates vast datasets, from patient recruitment to safety monitoring, often residing in isolated databases. This fragmentation creates blind spots and hampers decision-making.
However, end-to-end visibility is more than data integration; it’s about having a comprehensive view of the entire clinical trial landscape. This approach empowers stakeholders at all levels to proactively identify risks, refine strategies, and make data-driven decisions in real-time.
However, end-to-end visibility is more than data integration; it’s about having a comprehensive view of the entire clinical trial landscape. This approach empowers stakeholders at all levels to proactively identify risks, refine strategies, and make data-driven decisions in real-time.
The Power of Advanced Data Analytics
At the core of achieving end-to-end visibility is advanced data analytics. These tools can process large datasets, analyze intricate relationships, and extract valuable insights. Sophisticated algorithms and statistical models can predict potential issues, improving resource allocation and patient safety.
For instance, predictive analytics can forecast patient recruitment rates, while machine learning algorithms can detect adverse events early. These capabilities are vital as clinical trials become more global and complex.
For instance, predictive analytics can forecast patient recruitment rates, while machine learning algorithms can detect adverse events early. These capabilities are vital as clinical trials become more global and complex.
Customized Visual Dashboards: A Window into Insights
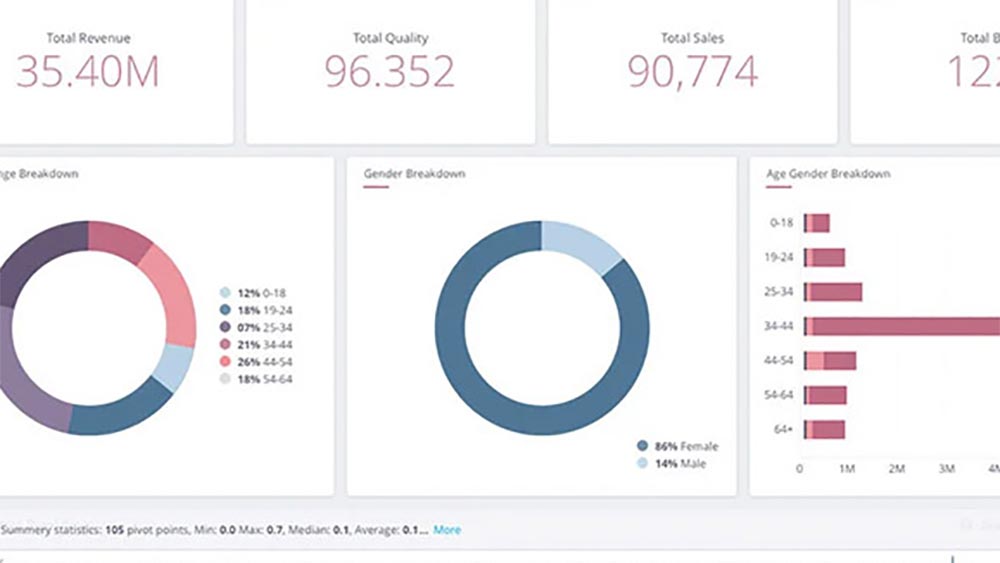
Customized visual dashboards are more than just data presentation tools; they are the windows through which sponsors gain real-time access to invaluable insights. These user-friendly interfaces provide dynamic displays of complex data, offering real-time updates and customizable views. What sets them apart is their ability to enable sponsors to break down data silos and synthesize massive volumes of disparate data points into one single source of truth that reveals actionable insights. This breakdown of data silos fosters collaboration, enhances transparency, and empowers stakeholders at all levels to make data-driven decisions with confidence.
Imagine a clinical trial manager tracking patient enrollment on a real-time dashboard, while a safety officer monitors adverse events on the same platform. Customization ensures stakeholders see precisely what they need to make informed decisions.
Imagine a clinical trial manager tracking patient enrollment on a real-time dashboard, while a safety officer monitors adverse events on the same platform. Customization ensures stakeholders see precisely what they need to make informed decisions.
The Future of Clinical Trials: Data-Driven Visibility
The future of clinical trials revolves around data-powered, end-to-end visibility. The benefits are compelling: shorter timelines, enhanced patient safety, cost reduction, and better decision-making. Regulatory bodies are also beginning to support the use of advanced analytics and dashboards in clinical trials.
In conclusion, achieving end-to-end visibility in clinical trials is not just a possibility; it’s a necessity in today’s complex pharmaceutical landscape. By leveraging advanced data analytics and customized visual dashboards, sponsors can confidently navigate modern trial challenges. The organizations that embrace this data-driven paradigm will lead the way in medical innovation.
In conclusion, achieving end-to-end visibility in clinical trials is not just a possibility; it’s a necessity in today’s complex pharmaceutical landscape. By leveraging advanced data analytics and customized visual dashboards, sponsors can confidently navigate modern trial challenges. The organizations that embrace this data-driven paradigm will lead the way in medical innovation.